Qualitative Analysis of Elements
The most commonly occurring elements in organic compounds are carbon, hydrogen, oxygen, nitrogen, sulphur and halogen elements. There is no direct method for the detection of oxygen. For detecting nitrogen, sulphur and halogens, we can use the sodium fusion test (Lassaigne’s test).
Sodium Fusion Test
This test is used for the qualitative analysis of elements nitrogen, sulphur and halogen in Organic compounds. In order to detect them, it is necessary to convert them into ionisable inorganic substances.
1) Test for Nitrogen
We can detect cyanide ion and hence, nitrogen ion in the sample by the Prussian blue test. The filtered alkaline solution resulting from the action of water upon the sodium fusion is treated iron (II) sulphate and thus, forms sodium hexacyanoferrate (II).
Upon boiling the alkaline iron (II) salt solution, some iron (III) ions are insensibly produced by the action of air. Now, we add dilute sulphuric acid to dissolve the iron (II) and (III) hydroxides. The hexacyanoferrate (II) reacts with the iron (III) salt, producing iron (III) hexacyanoferrate (II), Prussian blue. A Prussian blue precipitate or colouration indicates that nitrogen is present.
FeSO4 + 6NaCN → Na4[Fe(CN)6] + Na2SO4
3Na4[Fe(CN)6] + 2Fe2(SO4)3 → Fe4[Fe(CN)6]3 + 6Na2SO4
2) Test for Halogens (Nitrogen and Sulphur Absent)
We acidify a portion of the fusion solution with dilute nitric acid. We, then add an excess of silver nitrate solution. A precipitate indicates the presence of a halogen. We decant the mother liquor and treat the precipitate with dilute aqueous ammonia solution. If the precipitate is white and readily soluble in ammonia solution, chlorine is present. In case, it is pale yellow and difficulty soluble, bromine is present. If it is yellow and insoluble, then iodine and bromine may be confirmed by some more tests.
3) Test for Halogens (Nitrogen and/or Sulphur Present)
Cyanide and sulphide ions both interfere with this test for halide by forming silver cyanide and silver sulphide precipitates. If nitrogen or sulphur is present, we must remove the interfering ions. To remove cyanide and sulphide ions, we have to acidify the fusion solution with dilute nitric acid. Then, we have to evaporate it to half of the original volume to expel hydrogen cyanide and/or hydrogen sulphide which may be present.
Qualitative Analysis of Functional Groups
1) Alcoholic –OH group
We can detect the alcoholic group by the following tests:
- Sodium Metal Test: We conduct this test on the basis of the appearance of effervescence due to the liberation of hydrogen gas in reactions of sodium with alcohol.
2R – OH + 2Na → 2RONa + H2
- Acetyl Chloride Test: Acetyl chloride reacts vigorously with primary and secondary alcohols with the evolution of hydrogen chloride. The hydrogen chloride gives white fumes of ammonium chloride with ammonium hydroxide.

- Ceric Ammonium Test: To the sample, we add a few drops of ceric ammonium nitrate and shake well. The appearance of pink or red colour indicates the presence of an alcoholic group.
2ROH + (NH4)2Ce(NO3)6 → (ROH)2Ce(NO3)4 + 2NH4NO3
2) Carbonyls (Aldehydes and Ketones)
- 2,4-dinitrophenyl hydrazine test: We add a small amount (2 drops or 0.05 – 0.1g) of the substance to 3 ml of 2,4-dinitrophenyl hydrazine reagent and shake well. A crystalline precipitate indicates the presence of a carbonyl compound. Occasionally the precipitate is oily at first but this becomes crystalline upon standing.
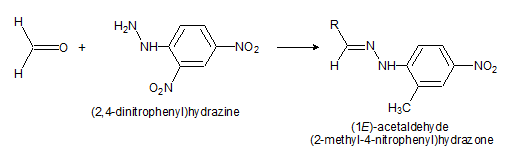
3) Aldehydes
- Schiff’s Test: We dissolve the given compound in alcohol and then add 1-2ml of Schiff’s reagent. The appearance of pink, red or magenta colour confirms the presence of aldehyde group.
- Tollen’s Test (Silver Mirror Test): We add 3-4 drops of the liquid to the Tollen’s reagent. We heat the container. A shining mirror precipitate confirms the presence of the aldehyde.
2Ag(NH3)2+ + RCHO + 3OH– → RCOO– + 2Ag¯ + 4NH3 + 2H2O
4) Carboxyl Group
We can identify Carboxylic acid by the following tests:
- Sodium Bicarbonate test: We add Sodium bicarbonate (NaHCO3) to the 1 ml of the sample. A pinch of effervescence indicates the presence of a carboxylic group.
RCO2H + NaHCO3 → RCOONa + CO2 + H2O
- Ester Test: We warm a small amount of the acid with two parts of absolute ethanol and one pare of concentrated sulphuric acid. We cool the solution and pour it continuously into aqueous Na2CO3 solution. A sweet, fruity smell of an ester confirms the presence of ester.
5) Amino Group
The most important basic nitrogen compounds are the primary, secondary and tertiary amines and they dissolve in mineral acids and change red litmus to blue.
- Chemical classification of the amine function: The classification of primary, secondary or tertiary amines is done by means of the reaction with nitrous acid.
- Nitrous Acid Test: We add 2g of the substance to 5 ml of 2 M HCl acid. Then, we cool it and add 2 ml of ice-cold 10% aqueous NaNO2 solution slowly by means of a dropper. If we obtain a clear solution, with a continuous evolution of nitrogen gas, the substance is a primary amine.
RNH2 + HONO → ROH + H2O + N2
Comments
Post a Comment